Meet the FDA’s traceability rule with a software that simplifies recordkeeping, automates supplier tracking, and keeps you audit-ready at all times. Capture, store, and share critical tracking events and key data elements without the manual chaos.
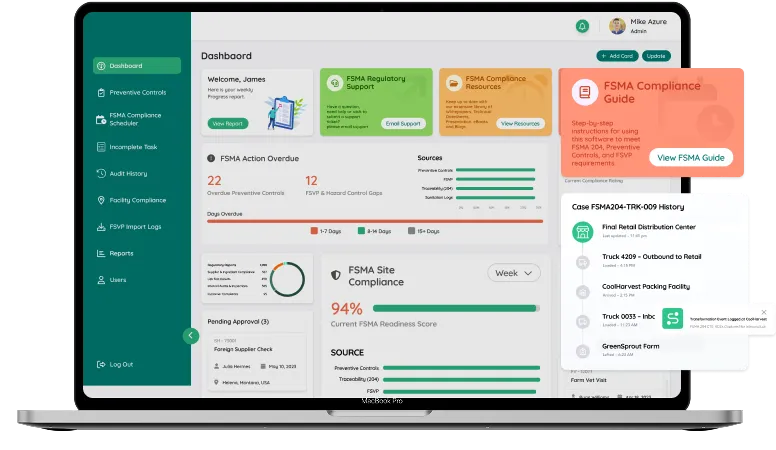























































FSMA 204 requires traceability records to be delivered within 24 hours in an electronically sortable format, which becomes nearly impossible when data lives in spreadsheets, paper logs, or siloed systems. Miss a KDE or delay a record, and you’re instantly exposed to regulatory risk.
Manual spreadsheets and paper logs make it impossible to maintain KDE accuracy and traceability consistency.
Suppliers follow different tracking formats, making it hard to consolidate data for FSMA traceability reporting.
Poorly connected systems make it nearly impossible to pull FDA-required data electronically within the 24-hour response window.
Missed events between receiving, transforming, and shipping cause compliance breakdowns.
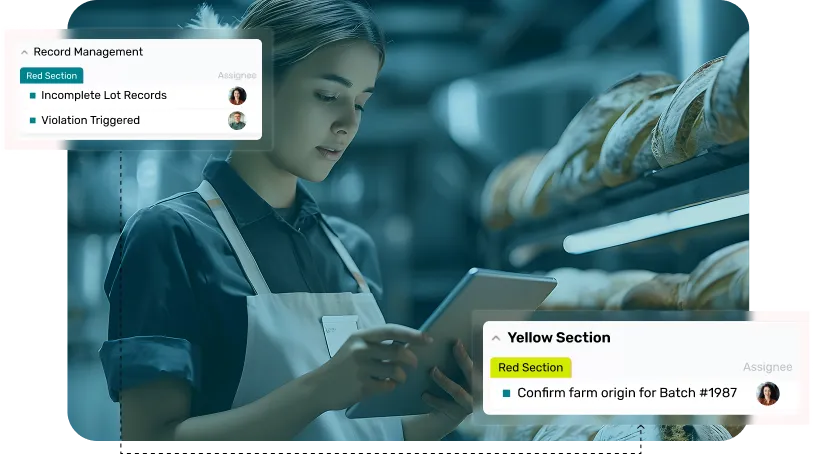
Digitize your traceability operations with an FSMA 204 compliance software that captures every event, consolidates supplier data, and generates compliance reports in seconds.
Ensure precise KDE documentation across all touchpoints, creating a living record of product history that satisfies FSMA 204 scrutiny and supports rapid investigation.
Generate and manage unique traceability lot codes for every movement event, ensuring clear lineage and rapid product isolation during investigations.
Produce FDA-formatted traceability reports on demand, giving inspectors immediate access to structured records without manual compilation or delays.
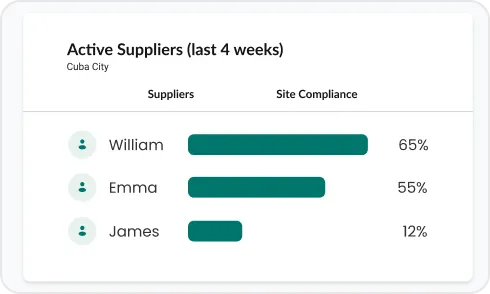
Unify inconsistent supplier records into a standardized format, delivering full end-to-end visibility while reducing onboarding and documentation friction.

Store all compliance data in a secure, cloud-based environment, ensuring controlled access, encrypted storage, and dependable version retention.
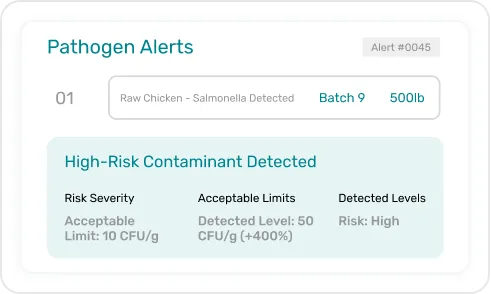
Monitor data integrity in real time with automatic alerts for missing KDEs or discrepancies, helping you prevent violations before they occur.
Gain unbroken oversight of every KDE from intake to distribution, forming a defensible audit trail that meets FSMA 204 expectations and elevates supply chain accountability.






Not every food business falls under FSMA 204, but for those on the Food Traceability List, traceability is mandatory, not optional. This section breaks down what FSMA requires for each covered category and how our platform aligns compliance with real operational workflows.
FSMA Targeted Foods: Cucumbers, herbs, leafy greens, melons, peppers, tomatoes, sprouts, tropical fruits, and fresh-cut vegetables.
Fresh produce moves fast, and FSMA 204 requires that every movement, from harvest to distribution, is traceable with precision. The FSMA 204 compliance software automates field-to-retail traceability while logging all KDEs and CTEs without slowing down daily operations.

FSMA Targeted Foods: Finfish, crustaceans, mollusks (raw, frozen, cured, or ready-to-eat).
Seafood supply chains are some of the most tightly regulated under FSMA 204. The FSMA 204 Compliance Software automates vessel-to-facility traceability, cold chain validation, and import/export documentation to ensure full chain-of-custody for every catch.

FSMA Targeted Foods: Soft cheeses, fresh cheeses, certain aged cheeses, shell eggs, and liquid egg products.
Cheese and egg products come with high microbial risk and strict temperature controls. FSMA 204 requires an unbroken traceability trail from raw intake to final packaging, and the platform centralizes every KDE to prove compliance at each stage.

FSMA Targeted Foods: Peanut butter, almond butter, tahini, cashew butter, and tree-nut or seed-based butters listed on the FTL.
Nut butter processing involves multiple transformation events and high allergen risk, making traceability mandatory under FSMA 204. The solution logs every ingredient, process, and shipment so you can prove origin and respond instantly during investigations.

FSMA Targeted Foods: Deli salads, sandwiches, prepared meals, cut fruit blends, sushi components, hummus, RTE dips, and high-risk refrigerated packaged foods.
Ready-to-eat production has no kill step, which makes traceability critical under FSMA 204. The FSMA 204 compliance software tracks every ingredient, process event, and shipment so you can prove compliance instantly during audits or recalls.

FSMA Targeted Foods: Any covered product received, stored, shipped, or sold that appears on the FTL, including produce, seafood, dairy/eggs, nut butters, and RTE products.
Distributors and retailers are the final checkpoint before food reaches consumers, which means traceability gaps here become direct liability. FSMA 204 requires accurate KDE logging at receiving and shipping, proof of cold chain integrity, and complete visibility into where each lot came from and where it went next.

Create a synchronized traceability view across your tech stack so you can verify product history, validate records, and respond to auditors with confidence.

























































Read what our clients say about their experiences and the difference our solutions have made for them.
We were extraordinarily pleased with the functionality and depth of understanding that Folio3's solution exhibited after a relatively brief but incisive, project kickoff meeting. Folio3 ``gets it`` from the start, relieving us from tedious development discussions drawn out over a long period of time.

Folio3 impressed me by learning a BI tool they did not have prior experience with and in a short amount of time produced analysis reports ready for business consumption. We are excited on the next set of critical reports Folio is working on for us and expect the relationship to continue for the foreseeable future.

"The team has done a tremendous job from testing and deploying our DAX applications to go live. They have also helped develop a reusable pattern for scheduling processes used by multiple workers. We will definitely continue to lean on folio3 for future support of these applications."

Folio3 has been an outstanding NetSuite implementation partner, excelling in complex integrations across our business units. Their expertise, innovative solutions, and responsive, supportive team make them truly impressive.

BioPak adopted NetSuite early in Australia, using its ERP, CRM, and eCommerce. After initial challenges with development partners, we partnered with Folio3 in 2016. Their transparency, work ethic, and seamless collaboration reignited our drive for crucial upgrades and innovations, making them a trusted partner in our growth.

We needed an online presence for our business that catered to both our B2B and B2C clientele and we needed it in record time. Folio3 was able to deliver in our timeline, in budget, and has continued to provide us with excellent on-going support.

We thank Folio3 for their dedication and hard work over the past 12 months. Folio3 team has been a great help to our organization, and I am proud of the relationship we have built between our colleagues and companies. I look forward to continuing to work with the Folio3 team.

Folio3 was extremely qualified within our NetSuite environment & was able to provide advanced proficiency when customizing the integration. I appreciate their ability to navigate the system well & provide the expertise needed to complete the project. We appreciate your hard work & look forward to our continued collaboration.

We are very excited to see the new PigWise app rolling out to production. When we had discussed the initial idea with Folio we had no idea that the final build would look this good. You guys rock!.

"I am happy to recogzine the work that was done by the Folio3 team. Our end users are very satisfied with the user interface and the performance of the app – and are excited to be more mobile. I appreciate the hard work and commiment to deliver a top quality solution. I look forward to continuing our business relationship."

"The Folio3 team has consistently exceeded our expectations. It felt as if we were working with an onshore team. It was their ability to understand our needs and keep us engaged throughout the entire process that has resulted in an exceptional product and a valued partner.."

FSMA 204 is the FDA’s Food Traceability Rule, requiring enhanced recordkeeping for high-risk foods on the Food Traceability List (FTL). Any business that manufactures, processes, packs, or holds these foods must comply, including importers and distributors.
The FTL includes produce, fresh-cut vegetables, soft and fresh cheeses, shell eggs, nut butters, ready-to-eat deli salads, seafood, and other high-risk products. If your product falls into one of these categories, FSMA 204 applies.
Key Data Elements (KDEs) are the specific data points required for traceability, and Critical Tracking Events (CTEs) are the moments in the supply chain when those data points must be captured. Together, they form the traceability chain the FDA relies on during investigations.
Businesses must be able to deliver a complete, electronically sortable traceability file within 24 hours, or sooner if requested during a public health emergency.
Yes. Imported products on the FTL are treated the same as domestic foods. The responsibility for accurate traceability applies to both foreign suppliers and U.S. receivers.
It automates KDE collection at every CTE, reduces manual recordkeeping, and centralizes documentation so all required traceability data is available in a single source of truth.
Yes. The platform generates and tracks lot codes, links them to supplier records, and maintains a continuous record of product movement from origin to distribution.
It can isolate affected batches in seconds, generate FDA-ready traceability reports, and help execute targeted recalls without halting unrelated inventory or production.
Not reliably. The FDA requires sortable electronic records, but manual spreadsheets are prone to errors, missing data, and gaps in CTE capture. An FSMA Software is the most dependable way to stay compliant at scale.
Non-compliance can result in warning letters, import refusals, injunctions, product seizures, loss of market access, or operational shutdown until traceability controls are fixed.