Today’s food manufacturers face daunting safety challenges: more recalls, tighter regulations, and very real human costs. Nearly 9.9 million Americans get sick from foodborne pathogens each year, and the industry bears staggering costs of ~$17.6 B annually. Every deviation, whether a labeling error, a contamination event, or a supplier defect, has the potential to compromise food safety and damage your brand reputation. That’s why consumers and regulators demand that you take every precaution.
This is where CAPA in the food industry becomes essential as a systematic process to identify food safety issues, correct them immediately, and prevent recurrence. Far from being a “nice to have,” CAPA is a cornerstone of modern food safety programs.
By embedding CAPA safety practices, you reduce the risk of foodborne illness, cut the likelihood of costly recalls, and preserve consumer trust in your products. Regulators view CAPA as critical: FDA guidance calls it “one of the most important quality system elements” for preventing repeat problems.
As a QA/QC manager, compliance leader, or plant manager, you know the pressure the food industry is under today. This guide shows how to apply CAPA to strengthen your operation and prevent food safety failures for continuous improvement.
What is CAPA in the Food Industry?
CAPA stands for Corrective and Preventive Action. In practice, it has two parts:
- Corrective Action: Steps to fix a detected problem and eliminate its cause. For example, quarantining adulterated products and fixing the faulty process. As per the FDA, corrective actions should correct the existing nonconformity and prevent it from happening again.
- Preventive Action: Steps to prevent potential problems before they occur. For example, updating cleaning procedures when data indicate an emerging contamination risk. FDA guidance emphasizes analyzing data for unfavorable trends and taking preventive actions
In a robust quality system, CAPA closes the loop on any deviation. Once you detect a nonconformance, you gather facts, fix the issue, and then monitor to ensure it never recurs. For example, suppose a HACCP critical limit is exceeded. In that case, a CAPA record is opened: you document the event, investigate its cause (root cause analysis), apply corrective steps, and update controls so it can’t happen again. Therefore, Auditors expect CAPA records as proof that you actively manage risks.
Regulatory and Industry Context for CAPA in the Food Sector
Food regulators and third-party auditors have clear expectations around CAPA. Each of these requirements means you must record and verify every CAPA step. Good documentation is often a key audit checklist item.
- FDA & FSMA: Facilities must have CAPA procedures. For instance, the FSMA Preventive Controls rule requires manufacturers to correct problems (and prevent recurrence) whenever a preventive control fails.
- HACCP (USDA/FSIS): HACCP regulations (9 CFR 417) mandate written corrective actions for any critical limit deviation. The plant must identify the cause, restore control, and ensure no adulterated product reaches consumers. All corrective actions must be documented in records.
- ISO 22000/GFSI: Food safety standards (ISO 22000, SQF, BRC, etc.) require documented CAPA as part of continuous improvement. These schemes explicitly tie CAPA to audit findings and HACCP deviations, ensuring that any food safety event triggers a formal action plan.
- Audits & Documentation: Inspectors will review CAPA records for completeness. FDA guidance emphasizes that CAPA activities be verified, documented, and communicated for management review. Missing or incomplete CAPA documentation can lead to audit observations or citations. Auditors expect evidence that CAPA outcomes were effective and have approved them.
When to Initiate CAPA: Common Triggers
You should initiate a CAPA as soon as a significant quality issue arises after any food sanitation and safety mistakes. Common triggers include:
- Audit or Inspection Finding: Any audit nonconformance or inspection finding requires a CAPA. Document a CAPA plan to investigate the root cause, apply corrective fixes, and prevent recurrence.
- Customer Complaint or Recall: Complaints of illness, foreign objects, or any recall event must spark a CAPA. Quarantine the product, identify the failure whether in process, ingredient, labeling, etc., and implement fixes and preventive measures.
- Out-of-Spec Laboratory Result: A failed QC test (microbiological, chemical, or physical) is an automatic CAPA trigger. Quarantine the lot, repeat testing as needed, and investigate the cause (equipment issue, contamination, recipe error, etc.).
- Process Deviation: If any process control (time, temperature, weight, etc.) goes out of specification, initiate CAPA. Even if corrected on the line, record the event, find why it happened (sensor failure, operator mistake), and update controls or training.
- Near-Miss or Trend: Recurring minor issues or near-misses (e.g., multiple small defects) signal a systemic risk. Start CAPA when you see a pattern: analyze the trend, determine contributing factors (equipment wear, material variance), and implement preventive fixes.
- Supplier Quality Issue: A supplier defect (e.g, contaminated ingredient, mislabel) should trigger CAPA on your supply chain. Investigate how it passed your controls, correct the immediate problem, and take preventive actions with the supplier (stricter specs, audits).
- Planned Change: Even planned changes (new equipment, ingredient source, process) should be evaluated. Consider launching a proactive CAPA: update hazard analyses, retrain staff, and verify that the change won’t introduce new risks.
In each case, logging the event and carrying CAPA to completion lets you resolve the issue and prevent repeats. Over time, it drives continuous improvement. Any problem that could affect food safety compliance should be captured in your CAPA system and addressed systematically.
The CAPA Lifecycle: Step-by-Step Process
Managing CAPA isn’t just about fixing problems; it’s about following a disciplined CAPA process that gives you complete visibility from detection to closure. By breaking the journey into clear CAPA process steps and documenting each one, you create a repeatable system that auditors trust and your team can follow with confidence.
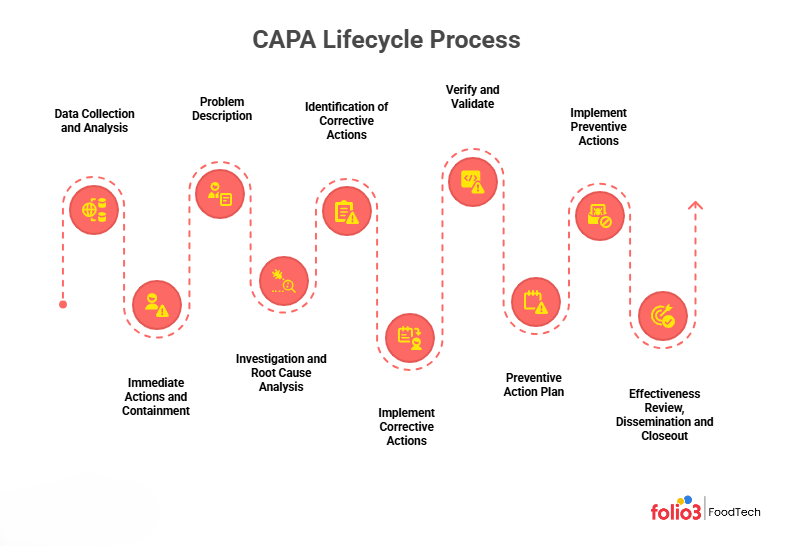
1. Data Collection and Analysis
Gather all relevant data about the issue: audit reports, lab test results, customer complaints, production logs, etc. Chart any trends (e.g., rising defect rates or recurring incidents). Analyze this information to understand the scope of the problem and its sources.
2. Immediate Actions and Containment
Take urgent steps to limit any harm. Quarantine affected product, halt production if needed, or issue a hold notice. Document all containment actions. These immediate “corrections” fix the current problem and prevent further impact while you proceed with a deeper investigation.
3. Problem Description
Clearly define what went wrong: when and where it was detected, and which products or processes were affected. Include specifics (dates, lot numbers, measurements) in a concise problem statement. This focused description sets the stage for the investigation. For example, “Batch #123 of Cheese A tested positive for pathogen on Line 5 at 10 pm, 8/1”).
4. Investigation and Root Cause Analysis (RCA)
Conduct a thorough investigation to find the actual root causes. Do not assume – use data, inspections, and personnel interviews. Tools like fishbone diagrams or the 5 Whys can help trace a problem to its origin. Involve a cross-functional team for diverse perspectives. As one industry expert emphasizes, perform an RCA for every CAPA to avoid merely treating symptoms. Document each finding and the actual root cause(s).
5. Identification of Corrective Actions
Based on the root cause(s), list corrective actions to eliminate the problem. This may include reworking or rejecting the affected product, repairing equipment, retraining staff, or changing process parameters. Ensure actions address both the specific issue and its underlying cause. For example, if an ingredient was mislabeled, a corrective action is to relabel the batch correctly.
6. Implement Corrective Actions
Assign an owner and a deadline for each corrective action. Execute the tasks and collect evidence of completion (e.g., signed work orders, calibration records, training logs) in the CAPA record. Update any relevant procedures or instructions to reflect the fix. By this point, the original issue should be resolved.
7. Verify and Validate
Confirm that your corrective actions actually solved the problem. Conduct retesting or re-inspection as appropriate. For significant changes, run validation tests (for example, verify that a new sanitizing procedure consistently eliminates the contaminant). Attach objective evidence (test results, inspection reports, photos) to the CAPA as proof of resolution.
8. Preventive Action Plan
Develop preventive measures so the issue doesn’t occur elsewhere. Ask if the root cause could affect other products, lines, or suppliers. For instance, if a cleaning lapse caused contamination on one line, schedule more frequent sanitation audits plant-wide. Write a preventive action plan with specific steps, owners, and deadlines.
9. Implement Preventive Actions
Execute the preventive steps. Update your HACCP/FSMS documents (hazard analyses, SOPs, training materials, supplier specifications) to include the new controls. Train staff on any changes. Assign and track ownership for each preventive task to ensure completion. Preventive actions might consist of adding new CCPs, tightening critical limits, or enhancing monitoring.
10. Effectiveness Review, Dissemination, and Closeout
Confirm the CAPA is complete and adequate. Monitor whether similar issues recur (for example, track defect or complaint rates). Summarize the CAPA actions and have management review them. Share lessons learned across the organization (for instance, in QA meetings or training sessions). Meanwhile, the CAPA findings and actions should be communicated to responsible personnel and included in management review. Once management accepts that objectives are met, formally close the CAPA. Completing this cycle closes the loop on the issue and reinforces continuous improvement.
Essential CAPA Tools and Methods for Food Safety
In food manufacturing, several quality tools support CAPA and continuous improvement. Each of these tools helps you analyze problems, plan solutions, and quantify risk, all integral to an effective CAPA process improvement.
Root Cause Analysis (RCA)
A systematic method to find why a problem occurred. Common techniques include the “5 Whys,” Fishbone (Ishikawa) diagrams, and 8D reports. RCA ensures you address fundamental causes, not just symptoms.
Fault Tree Analysis (FTA)
A top-down, logic-based diagram that starts with a potential hazard or failure (e.g., a safety incident) and works backward to identify all possible underlying causes. It is helpful for complex failures, such as tracing how multiple equipment failures could lead to contamination.
Failure Mode and Effects Analysis (FMEA)
A proactive approach to identify where and how a process might fail (failure modes), assess the severity, occurrence, and detection of each, and prioritize fixes. Many plants use FMEA on production processes or new product introductions to preempt potential defects.
Risk Assessment Tools
Methods like HACCP hazard analysis, risk matrix, or control banding help you assess food safety risks. For example, HACCP requires identifying hazards at each step and setting controls. Integrating HACCP analysis with CAPA ensures that any new risk (as revealed by CAPA findings) is added to your preventive controls.
CAPA Documentation for Measuring Effectiveness
Thorough documentation is the backbone of an effective CAPA program. It lets you measure whether CAPAs are completed on time and truly fix problems. In fact, HACCP regulations explicitly require that all corrective actions be documented in records. Proper CAPA documentation lets auditors verify your process and demonstrates continuous improvement.
Each CAPA record should capture key fields and actions, including:
- Incident ID: A unique identifier for tracking the event.
- Date/Time Reported: When the issue was detected or reported.
- How Detected: The source (e.g., customer complaint, internal audit, lab failure, equipment alarm).
- Severity/Impact Score: A risk rating of how serious the issue is (often a numeric score or category).
- Immediate Actions Taken: Any containment or corrections done right away (e.g., product hold, line shutdown).
- Root Cause(s) and Analysis Method: The underlying causes identified (from RCA), plus the method used (e.g., “5 Whys” or fishbone).
- Proposed Corrective/Preventive Actions: The planned steps to fix the issue and prevent recurrence.
- Action Owner(s) and Due Dates: Who is responsible for each task and its deadline.
- Verification Evidence: Records showing actions were implemented (photos, re-test results, signatures).
- Closure Criteria: What conditions must be met to close the CAPA (e.g., retest passed, no recurrence for 3 months).
- Management Review Comments: Notes from managers reviewing the CAPA results and verifying effectiveness.
CAPA Metrics & KPIs for Food Manufacturers
Tracking CAPA metrics turns documentation into insight. Analyzing these metrics quantifies CAPA effectiveness and enables management to track performance and drive continuous improvement. Important KPIs include:
- Mean Time to Close CAPA: The average number of days from CAPA opening to closure. A lower number indicates a faster, more responsive system. Industry best practice is to close CAPAs within about 60 days, as this metric directly reflects process efficiency.
- Recurrence (Repeat Issues): The percentage of problems that reappear after a CAPA. A high recurrence rate means root causes were not fully addressed. Counting reopened CAPAs or repeat incidents highlights gaps in your investigations.
- % CAPAs Verified Effective: The fraction of CAPAs with completed verification steps (i.e., proof that the fix worked). A low percentage indicates skipped follow-up. Auditors expect most CAPAs to have documented verification. Tracking it ensures CAPAs aren’t closed prematurely.
- % Overdue Actions: The share of CAPA tasks past their due date. Overdue actions stall the process and indicate resource bottlenecks. Keep this low (e.g., <10%). Monitoring overdue CAPAs helps you prioritize workload and resources.
- Cost per CAPA: Total resources spent on a CAPA (labor hours and materials). While harder to measure, estimating costs (e.g., hours spent by staff) provides insight into impact. This KPI can justify improvements: if CAPAs are costly, it underscores the value of stronger preventive measures.
- Audit Nonconformance Trend: Track the number or trend of CAPA-triggering nonconformances found in audits (internal or external) over time. The goal is a downward trend. Fewer new audit findings indicate your CAPA system is improving compliance.
Regularly reviewing these KPIs helps you see if CAPA performance is improving over time. For each KPI, set targets (for example, 90% of CAPAs verified or <5% recurrence) and monitor them in management reviews. As a result, you can ensure CAPAs truly drive process improvement and keep your safety program on track.
CAPA in Action: Real-World Examples in the Food Industry
These examples illustrate how CAPA plans translate incidents into corrective and preventive measures, safeguarding food safety and compliance.
Example: Environmental Pathogen Outbreak
A ready-to-eat salad producer found Listeria in a final-product test. The suspect batches were immediately recalled and destroyed. The CAPA team investigated and identified the root cause: a sanitation gap in a wash tank. Corrective actions included a complete equipment deep-clean and retraining of sanitation staff.
As a result, preventive actions were then applied plant-wide, like revising the cleaning procedure and increasing environmental swabbing frequency. This follows a textbook CAPA approach: recall the contaminated food and adjust sanitation. As a result, the company not only eliminated the current contamination but also prevented similar issues on other lines.
Example: HACCP Deviation in Pasteurization
At a dairy processor, a pasteurizer malfunction caused milk to fall below the required kill-step temperature (a critical HACCP control). The affected batch was held and later safely reprocessed after fixing the issue. The CAPA investigation found a failed temperature sensor as the root cause. Corrective steps were to replace the sensor and recalibrate the equipment.
Preventively, the plant tightened maintenance schedules and retrained operators to monitor critical sensors closely. By fixing the immediate error and strengthening the process, this CAPA closed the loop on the HACCP failure. Auditors saw that the company not only addressed the deviation but also improved its control measures.
Common CAPA Pitfalls & How to Avoid Them (200 words)
Even a solid CAPA process can fail in practice. Common pitfalls include:
- Treating Symptoms Instead of Causes: A frequent mistake is applying a quick correction without investigating the root cause. For example, simply retraining staff after a mistake, without finding why the error happened. To avoid this, insist on a thorough root-cause analysis for every CAPA. Engage a team, gather data, and confirm the real cause before deciding on actions.
- Skipping Verification: Closing a CAPA without verifying effectiveness is risky. Some teams stop after implementing fixes and never check the results. To prevent this, always set and meet verification checkpoints (e.g., retesting, audits) and document the evidence so an auditor can see it.
- Incomplete Documentation: Missing or vague records are an auditor’s nightmare. If your CAPA form lacks details (who did what, when, with what results), the CAPA is as good as undone. Ensure every CAPA is fully documented with all required fields, and consider using a checklist or software form so nothing is left out.
- Lack of Ownership and Follow-Up: CAPAs stall when no one is responsible or deadlines slip. Prevent this by assigning a single owner for each CAPA and precise due dates. Managers should track progress and hold people accountable.
By avoiding these pitfalls and reinforcing RCA, verification, documentation, and accountability, your CAPA process will be more effective and audit-ready.
Digitize CAPA with Folio3 FoodTech’s Safety & Compliance Suite
As part of Folio3 FoodTech’s comprehensive Food Safety suite, our digital solutions make CAPA easier and more effective. Here’s what you gain by leveraging our software solutions:
Automated CAPA Workflows: Using our food quality management module, you can log CAPA issues with a click, assign tasks to team members, set due dates, and attach evidence. Automated reminders and dashboards help you track each CAPA through the 10-step lifecycle, ensuring no action is missed.
Integrated Compliance & HACCP: Our compliance management tools (FSMA and HACCP modules) tie CAPA directly into your preventive controls and HACCP plans. A CCP deviation or FSMA compliance gap can automatically spawn a CAPA record. Likewise, any CAPA completion updates your HACCP hazard analysis, keeping your plans current. Everything is audit-ready with digital records and version control.
Recall & Traceability Integration: In the recall management and traceability modules, CAPA is part of rapid response. If a recall occurs or a supplier issue emerges, data from our traceability system (batch codes, supplier lots) auto-populates CAPA forms. This speeds root-cause analysis and lets you quickly target affected products or raw materials.
Analytics & Dashboards: Real-time KPIs and reports let you measure CAPA performance instantly, average closure times, overdue actions, and more – all within the platform. By digitizing CAPA with Folio3 FoodTech, you get a streamlined, connected solution that enforces best practices, captures evidence for audits, and drives continuous improvement across your operation.
Conclusion
CAPA is indispensable for safeguarding food safety, ensuring compliance, and driving continuous improvement. By following the 10-step CAPA process outlined above, you can close gaps in your HACCP and FSMS, reduce contamination risks, and avoid costly recalls. Assess your current CAPA system: do you have defined workflows, complete documentation, and verification? Close any gaps by training your team and using the right tools.
Ready to build a stronger CAPA program? Contact us for expert consultation or book a demo to get started. Let’s strengthen your food safety together
FAQs
What’s the Difference between Correction, Corrective Action, and Preventive Action?
Correction fixes an immediate non-conformance (e.g., segregating a mislabeled product).
Corrective Action addresses the root cause of an existing issue so it doesn’t recur (e.g., retraining staff and updating SOPs).
Preventive Action eliminates potential problems before they occur (e.g., adding label verification software).
How Do You Choose the Right Root Cause Method (5-Why vs Fishbone)?
Use 5-Why when the problem is simple and you need to drill down quickly.
Use a Fishbone Diagram for complex issues with multiple possible contributing factors (people, process, equipment, environment).
What Documentation Is required to close a CAPA during an Audit?
Auditors expect to see: incident details, root cause analysis, corrective and preventive actions taken, assigned owners, verification evidence, closure criteria, and management review notes. Incomplete documentation is a red flag.
How Can Software Reduce CAPA Cycle Time and Recurrence Rates?
Food safety software automates CAPA workflows, assigns tasks with reminders, centralizes records, and integrates with HACCP/FSMA modules. It reduces closure time, improves accountability, and lowers recurrence through better trend analysis and monitoring.
What are Strong Examples of HACCP Corrective Actions at CCPs?
Reprocessing or discarding under-cooked product.
Adjusting pasteurization time/temperature and verifying with calibration records.
Holding and retesting a lot that failed a CCP limit (e.g., pathogen test).
How do I Prioritize CAPAs Using Risk Assessment (FMEA)?
Apply FMEA by scoring each issue on Severity, Occurrence, and Detectability. Multiply the scores to calculate a Risk Priority Number (RPN). High-RPN issues should be prioritized for immediate CAPA action.







