As a food processor or safety manager, you know contaminated products can wreak havoc on public health and profit. In fact, CDC data show roughly 9.9 million foodborne illnesses and over 53,000 hospitalizations in the U.S. each year. Plus, the recall costs are steep for food manufacturers and processors, averaging ~$10 million in direct costs per incident.
FSMA Section 204 (the Food Traceability Rule) addresses this risk by requiring detailed tracking of designated high-risk foods. As per the FDA, these new traceability requirements should allow faster identification and rapid removal of potentially contaminated food from the market, resulting in fewer foodborne illnesses.
Under FSMA 204, you must capture and share key data elements at each step of the supply chain, so that if a problem arises, you can quickly isolate and remove the contaminated lot. In this guide, you’ll learn what FSMA 204 traceability requires, who it affects, and how to build a practical traceability plan.
Understanding FSMA & Section 204: Why Traceability Matters
Signed in 2011, FSMA gave the FDA the mandate to shift U.S. food safety from reaction to prevention. The agency now requires science-based hazard analyses and written preventive controls for food facilities. In short, FSMA is built on preventing hazards rather than responding after the fact. This preventive mindset underpins all FSMA regulations, including the new traceability rule.
Meanwhile, FSMA-certified operations already have the foundational systems needed to meet traceability standards. Learn more about FSMA Certification and how it supports compliance.
What is Section 204 (Food Traceability Rule)?
What is the FSMA traceability Rule 204? Section 204 of FSMA directed the FDA to create enhanced traceability for certain high-risk foods. The FDA published the final Food Traceability Rule (FSMA 204) in November 2022. It requires firms that grow, manufacture, pack, or hold foods on the FDA’s Food Traceability List (FTL) to keep extra records at specific steps. The rule spells out exactly what data (Key Data Elements) to record at each Critical Tracking Event, enforcing FSMA’s preventive approach.
Why Traceability is Critical for Business and Public Health
Faster, targeted recalls: Enables rapid, targeted recalls by identifying and removing only contaminated batches, minimizing financial and reputational losses.
Consumer trust and brand protection: Strengthens consumer trust by demonstrating transparent traceability in food safety and proactive risk management.
Regulatory compliance: Ensures regulatory compliance with FSMA 204 traceability, preventing costly violations and supply chain disruptions.
Supply-chain visibility: Improves supply chain visibility, helping you detect contamination or inefficiencies before they escalate.
Public health impact: Protects public health by reducing illness spread through faster contamination detection and response actions.
FSMA 204 Requirements & Key Concepts
There are several parameters regarding the requirements of FSMA 204 traceability that you must be aware of to ensure true compliance according to the rule.
Foods Covered by the Food Traceability List (FTL)
FSMA 204 applies only to specific high-risk foods on the FDA’s Food Traceability List. Key categories include:
- Dairy: Soft and semi-soft cheeses.
- Eggs: Shell eggs (chicken.
- Nuts: Nut butters (peanut and tree-nut spreads).
- Produce: Cucumbers; fresh herbs; leafy greens (whole and cut); melons; peppers; sprouts; tomatoes; tropical tree fruits; and fresh-cut fruits/vegetables.
- Seafood: Certain finfish, smoked finfish, crustaceans, and molluscan shellfish.
- Other: Refrigerated ready-to-eat salads (egg, seafood, pasta, etc.).
If your products contain any of these listed foods (in the same form), the rule applies to you. Foods not on the list are outside FSMA 204.
Critical Tracking Events (CTEs) and Key Data Elements (KDEs)
Under the FSMA 204 traceability rule, you’re required to record and maintain specific information at defined points as CTEs and KDEs in your supply chain. Together, they form the foundation of traceability in food safety, enabling rapid recall and investigation when contamination occurs.
Critical Tracking Events (CTEs)
CTEs represent every major event where food is moved, transformed, or altered in its journey from farm to consumer. Common examples of CTEs include:
- Harvesting: Capturing when and where raw agricultural commodities are collected.
- Cooling: Logging temperature control events immediately after harvest to maintain food safety.
- Initial packing: Recording packaging dates, facility identifiers, and assigned lot codes.
- Shipping and receiving: Tracking who shipped the product, to whom, and when it changed hands.
- Transformation: Documenting when raw ingredients are combined or processed into new products.
Each of these checkpoints ensures traceability throughout the lifecycle of high-risk foods listed on the Food Traceability List (FTL).
Key Data Elements (KDEs)
KDEs are the specific pieces of data you must collect at every CTE. They serve as digital breadcrumbs that enable quick backtracking if contamination or labeling errors occur. Typical KDEs include:
- Traceability lot code (TLC): A unique identifier linking each product to its source.
- Product description: Including commodity name, quantity, and packaging details.
- Location identifiers: Such as grower, packer, or distributor facility codes.
- Date and time of event: For precise tracking of when food was harvested, packed, or shipped.
- Reference documents: Purchase orders, bills of lading, or invoices verifying chain-of-custody.
Traceability Lot Codes & Recordkeeping
FSMA 204 also requires a Traceability Lot Code (TLC) for each covered lot. A TLC is a unique alphanumeric code that you assign to a product lot when you first pack it, first receive it (for seafood), or transform it into a new product. Every record for that lot must include the same TLC, so all pieces of information stay linked.
In your traceability plan, explain how you generate and manage these codes. All required records (paper or electronic) must be kept legible and protected, like in secure files or backed-up systems, so you can produce any lot’s full history to the FDA within 24 hours if asked.
Compliance Timeline & Recent Updates
The FSMA 204 Traceability Rule was published in the Federal Register on November 21, 2022, and became effective January 20, 2023. Covered businesses were initially expected to comply by January 20, 2026, but in 202,5 the FDA proposed a 30-month extension.
The new compliance date is now set for July 20, 2028. The FDA is using a single deadline for all firms. Meanwhile, the agency is rolling out guidance and tools to help you prepare. For example, in 2023, the FDA released new FAQs, webinars, and a traceability toolkit.
Even if enforcement is delayed, the smart move is to start planning now. Building your FSMA 204 system early will save time and protect public health in the long run.
Business Applicability & Exemptions
The FSMA 204 regulation applies to any business, whether domestic or foreign, that manufactures, processes, packs, or holds foods on the FDA’s Food Traceability List (FTL). If your operation handles listed items, you must comply with the rule’s record-keeping, lot-coding, and traceability requirements.
However, certain businesses may be fully or partially exempt from the food traceability regulations:
- Retail food establishments or restaurants with average annual food sales under $250,000 (adjusted for inflation) may be exempt.
- Small farms (especially produce farms) with very low sales or those that sell directly to consumers may qualify for exemption or modified requirements.
- Foods that undergo a full “kill-step” or otherwise don’t retain the covered FTL commodity in its original form may be excluded.
It’s essential that you assess which of your products, which sites, and which supply-chain roles fall under the rule or qualify for exemption, so that you’re not caught off guard when enforcement begins.
How to Build a FSMA 204 Compliance Strategy
Building a strong FSMA 204 compliance strategy is more than meeting regulations; it’s about protecting your brand, customers, and supply chain integrity. By planning early and aligning your people, processes, and systems, you’ll turn compliance into a competitive advantage.
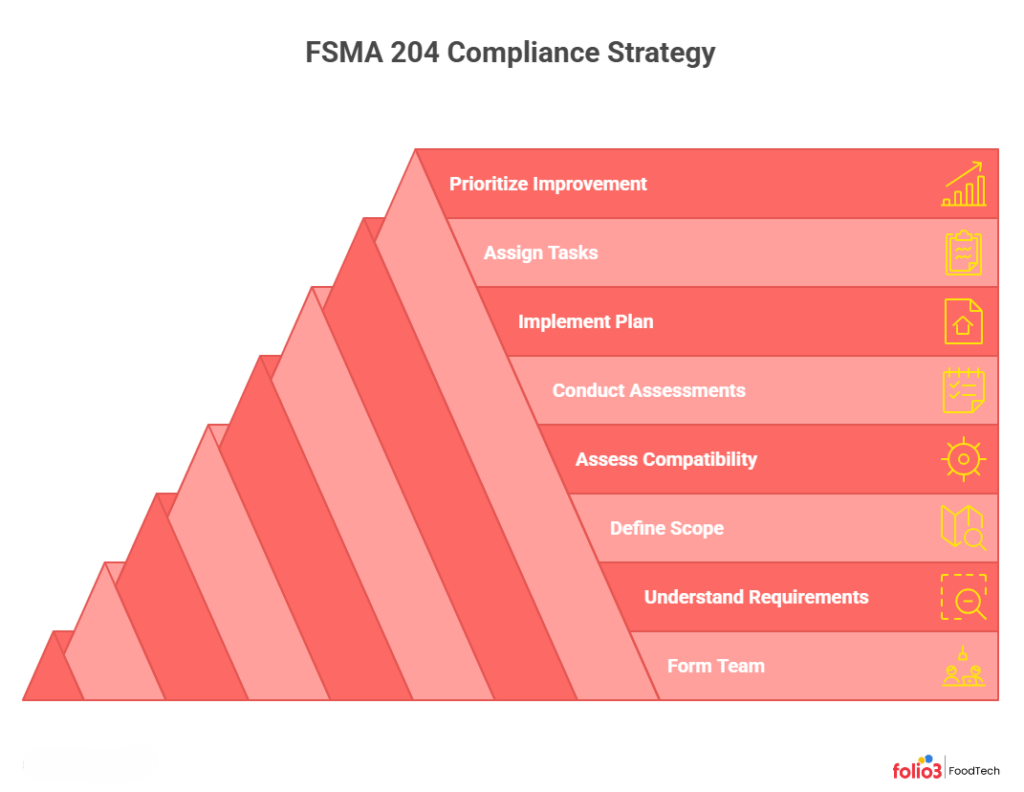
Step 1 – Form a Cross‑Functional Compliance Team
Assemble a team of key stakeholders: operations, QA/food safety, supply chain, procurement, IT, and management. Involving everyone who handles food or records data (from field workers to warehouse staff) ensures your plan fits real processes. This team will share responsibility for developing and overseeing your traceability program.
Step 2 – Get Familiar With FSMA 204 Requirements
Study the FSMA 204 final rule and support guidance together. Use official FDA resources (like the “Getting Started” document, webinars, and FAQs) to learn the terminology and data elements. Make sure everyone on your team understands what must be recorded at each step (the CTEs and KDEs).
Step 3 – Establish the Scope of Implementation
Identify exactly which of your products and sites are covered. List every FTL food you grow, process, pack, or hold, and note your role (e.g., farm, packer, distributor). Map your facilities and supply chain: determine where each CTE occurs in your operation and what data should be captured there. This scoped list of products and steps will guide your compliance plan.
Step 4 – Assess Traceability & Data Compatibility
Review your current traceability processes and data systems. Check if existing labels, forms, or software capture the required KDEs at each CTE. For example, can your packing-line labeler print lot codes and dates on every shipment? Do your electronic records include fields for supplier IDs and batch numbers? Note any missing data fields, manual workarounds, or mismatched formats that you’ll need to address.
Step 5 – Conduct Internal Gap & Risk Assessments
Compare your current state to the FSMA 204 requirements. List any gaps you find (for instance, no lot-code system at harvest or incomplete receiving logs) and assess the risk of each. Focus first on the highest-risk foods and steps. Prioritize fixes that eliminate the biggest vulnerabilities in your chain. Document your findings in a gap analysis report so you clearly see what must change.
Step 6 – Implement the Compliance Plan
With gaps identified, take action. Update your procedures and equipment to capture the missing data. This might mean programming your ERP or traceability software to record new fields, installing barcode scanners or printers, or revising paper forms. Test each solution to confirm it captures all required KDEs at the right CTE. Train your team on the new workflows. Track progress against your plan so you stay on schedule.
Step 7 – Assign Tasks & Start Tracking
Delegate specific tasks for each CTE. For example, have harvesting crews record crop data and lot codes, packing staff label cases, and warehouse personnel log shipments. Use checklists or system alerts to ensure no step is missed. Then begin real-time data collection: assign lot codes and record every required KDE for FTL products as you move through each event. Review the data regularly to catch and correct any errors.
Step 8 – Prioritize Continuous Improvement
FSMA 204 compliance is an ongoing process. Conduct mock recalls or traceability drills to test your system and find weak points. After each test or actual incident, update your plan and retrain staff on lessons learned. Monitor new products, suppliers, or tech updates and adjust your procedures. In short, treat traceability as a living program; continually improving it will pay off in faster responses and safer food long term.
Developing a Traceability Plan & Recordkeeping
Creating a well-defined traceability plan is the foundation of FSMA 204 compliance. Under the FSMA traceability rule, you’re required to document how your organization captures, stores, and retrieves data across every Critical Tracking Event (CTE). Here’s how you can build a traceability plan that not only meets the FSMA 204 regulation but also improves operational visibility and recall readiness.
Essential Elements of a Traceability Plan
Your traceability plan should clearly define what you track, how you track it, and who’s responsible. Focus on these essentials:
- List all covered foods: Identify every item on the FDA Food Traceability List (FTL) that your company grows, packs, or distributes.
- Define responsibilities: Assign traceability roles to departments, procurement, production, QA, and logistics, to ensure accountability.
- Describe data capture methods: Explain whether you use manual logs, ERP integrations, barcodes, or IoT systems for recording Key Data Elements (KDEs).
- Outline record storage and access: Specify where records are stored (digital or physical), who can access them, and how retrieval occurs within 24 hours if the FDA requests it.
- Include procedures for updates: Document how you’ll review and update the plan annually or when new products/processes are added.
Record-Keeping Best Practices
Accurate recordkeeping is the backbone of the FSMA 204 regulation. Keep your system simple, consistent, and audit-ready.
- Organize by traceability lot code: Group all records (harvest, packing, shipping, receiving) under one unique lot number.
- Ensure data integrity: Verify that every record is dated, signed (or digitally approved), and backed up in secure storage.
- Maintain retention standards: Keep all FSMA-required records for at least two years, as mandated by the FDA.
- Use searchable formats: Store records in formats that allow quick retrieval (spreadsheets, PDFs, or cloud databases).
- Conduct regular mock audits: Test your team’s ability to retrieve full traceability records quickly and accurately.
Assigning & Managing Traceability Lot Codes
Lot codes are the anchor of your FSMA traceability rule program; they connect every movement and transformation.
- Create unique identifiers: Assign a new lot code at the first instance of packing or processing an FTL item.
- Use standardized formats: Incorporate product, facility, and date details in each code for clarity and traceability.
- Record code assignment procedures: Document who generates lot codes, how they’re applied to packaging, and how errors are corrected.
- Link codes across supply chain partners: Share lot information with suppliers and customers for seamless traceability.
- Automate where possible: Use digital systems or barcode scanners to prevent duplication and improve data accuracy.
Technology & Tools for FSMA 204 Compliance
Adopting the right technology is key to simplifying FSMA 204 compliance. Modern digital tools help you capture, store, and share traceability data seamlessly across every point in your supply chain.
Digital Traceability Systems
Many companies use dedicated traceability software or cloud platforms to meet FSMA 204. These systems link production, inventory, and shipping data so KDEs are recorded in real time. Industry emphasizes it as a strong sense of urgency to embed more digital, tech-enabled traceability in the food supply chain.
For example, a cloud platform can capture a lot of code as soon as a shipment leaves the packing line. If you already use a food safety or food ERP system, check whether it offers an FSMA 204 module or integration.
EDI & GS1 Standards
Electronic Data Interchange (EDI) and GS1 standards are common tools for traceability. GS1 issues global identifiers (GTIN for products, GLN for locations) that map directly to the FDA’s CTEs and KDEs.
By scanning GS1 barcodes and sending EDI shipping notices or invoices, you can automatically exchange key lot and product data with trading partners. This standardized data exchange minimizes manual entry and errors.
Emerging Technologies & Future Solutions
New digital tools are reshaping compliance under the FSMA 204 regulation, making traceability faster and more reliable.
- Blockchain systems create tamper-proof, shared batch records across suppliers for transparent traceability.
- IoT sensors like temperature, GPS, RFID, and NFC tags automatically log Critical Tracking Events (CTEs) in real time.
- The FDA’s Low/No-Cost Traceability Challenge recognized blockchain and IoT as breakthrough traceability solutions.
- RFID/NFC tags now help farms scan and track products instantly, minimizing manual errors.
- AI-driven analytics detect anomalies, forecast risks, and optimize recall responses.
Benefits Beyond Compliance: Building Trust & Competitive Advantage
FSMA 204 traceability isn’t just a regulatory checkbox; it’s your opportunity to build consumer trust, strengthen brand reputation, and unlock new market opportunities through transparency and efficiency.
Building Consumer Confidence
Traceability under FSMA 204 not only protects consumers but also reassures them. A survey found 59% of consumers hesitate to buy a brand after a recall, and 60% avoid the entire category. By enabling faster, targeted recalls, FSMA 204 traceability helps minimize such fallout. When you can pinpoint and remove only the contaminated lot, you keep most of your product on shelves and restore confidence more quickly.
Efficient Recalls & Brand Protection
Well-implemented traceability means smaller, faster recalls, which saves money and limits brand damage. Instead of stopping an entire product line, you need only pull the affected batch. That containment protects your bottom line and reputation. It also streamlines any investigations or audits, since complete records are on hand. In effect, investing in FSMA 204 readiness today shields your business from the much greater costs of a large-scale recall.
Competitive Differentiation
Robust traceability can be a market advantage. Many retailers and buyers now demand strict traceability. Being FSMA 204-compliant shows you meet high safety standards, helping you win contracts. It also facilitates exports, as more countries expect proof of traceability. In short, strong traceability demonstrates quality leadership, helping you stand out in a crowded marketplace.
Take the Action
FSMA 204 traceability is now the law, so it’s time to take action. Start by reviewing the rule’s requirements for your products and writing a simple plan. Engage your cross-functional team to assign roles and implement tracking. Invest in systems or tools (even basic ones) to capture the required data. Then practice by running mock recalls or audits. This isn’t just about avoiding penalties; it’s an opportunity to strengthen your operations. By preparing now, you ensure your food supply chain and your customers are safer in the future.
To ensure the optimal application of your food process right according to rules, you can consult with our Foodtech experts to leverage an FSMA compliance software or book a free demo to see how it works for you.
FAQs
Is FSMA Different from HACCP?
Yes. HACCP focuses on identifying and controlling hazards in food production, while the FSMA traceability rule emphasizes prevention and documentation across the entire supply chain. FSMA goes beyond HACCP by requiring proactive traceability and accountability from farm to fork.
What Happens if You Fail to Comply with the FSMA 204 Traceability Rule?
Non-compliance can lead to FDA enforcement actions, product recalls, penalties, or import holds. More importantly, it risks consumer safety and damages your brand’s credibility and market trust.
Does the FSMA 204 Traceability Rule Apply to Imported Foods?
Yes. The FSMA 204 regulation applies equally to domestic and imported foods on the FDA’s Food Traceability List (FTL). Importers must maintain and provide traceability records just like U.S. producers and distributors.
How Long Must You Keep Records Under the FSMA Traceability Rule?
You must retain required records for at least two years and be able to provide them to the FDA within 24 hours of a request during audits or investigations.
How Can an FSMA Compliance Software Help You Comply with the 204 Traceability Rule?
An FSMA compliance platform automates recordkeeping, lot tracking, and data sharing across your supply chain. It reduces manual errors, ensures real-time visibility, and simplifies audit-readiness under the FSMA 204 traceability requirements.







