Food safety is no longer a box to tick; it’s now a prerequisite for doing business. Retailers like Walmart, Costco, and many foodservice chains increasingly require Safe Quality Food (SQF) certification from suppliers to mitigate liability and protect consumers. At the same time, food recalls and outbreaks continue to shake the industry as a single recall costs food companies around US$10 million in direct expenses, and even more when reputational damage and lost sales are considered.
For manufacturers, co‑packers, ingredient suppliers, logistics providers, and packaging producers, SQF certification has become a strategic investment rather than a voluntary badge.
This 2025 guide gives you a clear roadmap to obtaining and maintaining SQF certification with a step‑by‑step implementation plan, including relevant parameters. Use it as a blueprint to build a robust, cost‑effective food safety system and download our free gap‑analysis template to kick‑start your journey.
What is SQF? SQF Meaning in the Food Industry
Safe Quality Food (SQF) is a comprehensive food‑safety and quality management system recognised by the Global Food Safety Initiative (GFSI). In plain English, SQF is a set of requirements (the SQF Code) and assessment processes that help food businesses identify hazards, implement risk‑based controls, and demonstrate compliance through third‑party audits.
The program is administered by the SQF Institute, part of FMI – The Food Industry Association, and its codes are updated regularly to reflect regulatory changes and lessons learned from audits. Unlike minimal compliance programs, SQF covers the entire supply chain “from farm to fork”: primary production, manufacturing, packaging, storage/distribution, retail, and foodservice. Because it is benchmarked by GFSI, an SQF certificate is accepted globally by retailers and buyers.
Where SQF Sits in the Food Safety Landscape
SQF is unique in two ways. First, it is currently the only GFSI‑recognised scheme that encompasses every sector of the food supply chain. Second, it offers a modular structure, allowing companies to select the code that matches their sector (e.g., manufacturing vs. distribution) and maturity (entry‑level vs. fully benchmarked).
More than 13,000 sites across six continents are certified to SQF, reflecting its broad adoption. The program emphasises Hazard Analysis and Critical Control Points (HACCP) and preventive controls, aligning with regulatory requirements like the U.S. Food Safety Modernization Act. Throughout this guide, you’ll see how SQF fits alongside other food‑safety schemes and why major retailers view it as a credible assurance of due diligence.
Food Safety Programs & Codes
The SQF Food Safety Programs offer scalable certification paths from building basic GMP systems to achieving full GFSI-benchmarked compliance.
SQF Fundamentals Program (Entry Level)
Designed for small and medium‑sized businesses or start‑ups, the SQF Fundamentals Program helps companies implement a basic food‑safety management system built on the GFSI Global Markets Program. It focuses on core Good Manufacturing Practices (GMPs), simplified HACCP, and management commitment. The program acts as a stepping stone; certificates are issued without a score, and improvement is measured by non‑conformances instead of numerical grades. There are two tiers (Basic and Intermediate) to support progression toward full SQF certification.
SQF Food Safety Program (Main Certification Route)
The SQF Food Safety Codes are fully GFSI‑benchmarked and therefore meet retailer requirements. They apply a risk‑based HACCP methodology and provide detailed guidance for 17 industry sectors, including food manufacturing, pet food, storage and distribution, and packaging. Edition 9 of the code, published in 2020 and effective since May 2021, is currently the applicable version.
SQF Quality Program (Optional Quality Add‑on)
This code goes beyond food safety to address quality threats such as product specifications, consistency, and consumer satisfaction. It uses a risk‑based approach similar to HACCP and requires continuous monitoring of process parameters from raw materials to finished goods. Sites certified to the Quality Code may display the SQF Quality Shield on packaging.
Codes by Sector
SQF Codes are tailored to specific supply‑chain segments.
- The Manufacturing Code covers food processing facilities and pet‑food producers.
- The Packaging Code targets manufacturers of food‑contact materials.
- The Storage & Distribution Code applies to warehouses, cold storage, and third‑party logistics providers.
Additional codes exist for Primary Production, Retail, and Foodservice. Choosing the right code involves using the SQF Code Selector (available via SQFI) to identify the modules relevant to your operation, ensuring that Module 2 (System Elements) is combined with the correct industry‑specific module.
Table – SQF Programs vs. GFSI status vs. Best fit
| Program | GFSI‑benchmarked? | Best for |
Fundamentals (Basic/Intermediate) | No–entry program | Start‑ups and small manufacturers wanting a gradual path to certification |
Food Safety Program | Yes | Any company seeking globally‑recognised certification (manufacturing, packaging, distribution, primary production) |
Quality Program | No (add‑on) | Companies aiming to demonstrate brand consistency and reduce quality complaints |
Who Needs SQF?
While voluntary in principle, SQF certification is increasingly a commercial necessity. You need it if you are:
- Food and beverage manufacturers supplying supermarkets or restaurant chains.
- Co‑packers and contract manufacturers must reassure brand owners of strict control over third‑party operations.
- Ingredient and additive suppliers seeking to integrate into global supply chains.
- Packaging manufacturers, especially those producing primary packaging that contacts food.
- Cold storage and 3PL providers, since distribution errors can compromise product safety.
- Retailers and foodservice companies want to protect brand reputation and comply with retailer mandates.
Global retailers and foodservice providers accept SQF as proof of due diligence and risk management. Without certification, gaining shelf space can be difficult. Even if your customers don’t explicitly demand SQF today, aligning your systems with the code reduces liability and positions your business for growth.
What’s Current Right Now: Edition 9 and the Path to Edition 10
Edition 9 of the SQF Food Safety Code remains the basis for audits in 2025. SQFI released Edition 9 in October 2020 with improved clarity and risk‑based requirements. Edition 10 was planned for release in 2024, but the SQFI has delayed publication while its benchmarking application to GFSI is under review until March 2026.
It means that Edition 9 will continue to be audited until the transition window opens, which will be at least six months after Edition 10 is finalized. Staying current involves monitoring SQFI updates and using official guidance to prepare for future changes. When Edition 10 arrives, expect expanded requirements for environmental monitoring and an emphasis on food‑fraud prevention.
Top Benefits of Safe Quality Food Certification
The benefits of obtaining SQF certification extend far beyond a certificate on the wall:
- Consumer protection & brand trust: A properly implemented SQF system reduces contamination and food‑borne illness.
- Market access: Retailers and foodservice operators around the world recognise SQF. SQF is accepted by retailers and foodservice providers worldwide and helps ensure products meet hazard analysis and HACCP principles.
- Fewer duplicate audits: As SQF is GFSI‑benchmarked and covers the full supply chain, it reduces the need for multiple customer‑specific audits.
- Lower recall risk & improved ROI: Recalls are expensive as manufacturers and retailers suffer millions as the average direct cost of a recall, not counting lawsuits and lost sales. Investing in SQF reduces the likelihood of such catastrophic events.
- Enhanced traceability & continuous improvement: SQF requires robust record‑keeping and supplier verification, making it easier to trace issues and improve over time.
SQF vs. Other GFSI Standards
Several GFSI‑recognized schemes exist; choosing the right one depends on customer expectations and your organisational culture. SQF, BRCGS and FSSC 22000 are the most common in North America.
The FSNS comparison explains that BRCGS is product‑focused and globally prevalent; it provides prescriptive requirements and is often preferred by UK retailers. SQF is flexible and HACCP‑based; major U.S. retailers like Walmart and Costco mandate it for suppliers. FSSC 22000 is ISO‑based and integrates with ISO 9001, making it attractive to companies seeking system alignment.
Table – Comparing GFSI‑benchmarked schemes
| Scheme | GFSI recognition | Best for | Audit cadence |
| SQF | Yes | Broad supply‑chain coverage; U.S. retailers and co‑packers | Annual; unannounced at least once every 3 cycles |
| BRCGS | Yes | Product‑focused manufacturing and packaging; UK and EU markets | Annual; unannounced option |
FSSC 22000 | Yes | Companies with ISO‑based systems, e.g., multinational manufacturers | Three‑year certification with annual surveillance |
Choosing between schemes depends on your customer requirements and whether you value flexibility (SQF), prescriptive standards (BRCGS), or ISO integration (FSSC 22000). Remember, all GFSI schemes require annual audits and unannounced assessments at least once every three years.
The SQF Food Safety Certification Roadmap: Step‑by‑Step
Implementing an SQF system can feel overwhelming. Breaking the journey into manageable steps helps teams maintain momentum and ensures nothing gets overlooked.
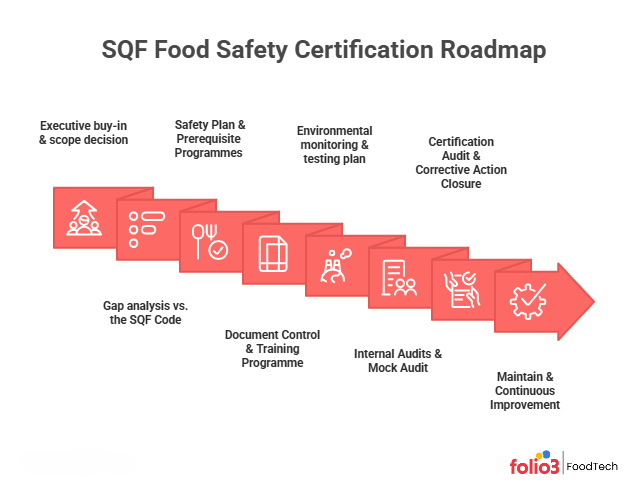
1. Executive Buy‑in & Scope Decision
Senior leadership must commit resources and set a clear scope. Decide which sites and product lines will be included and which SQF code applies (manufacturing, packaging, distribution, etc.). Appoint an SQF practitioner, a trained individual responsible for maintaining the system.
2. Gap Analysis vs. the SQF Code
Perform a systematic review of existing procedures, GMPs, and HACCP plans against the SQF Code requirements. Use a gap‑analysis tool or a consultant to identify missing elements. For Fundamentals participants, focus on building basic prerequisite programmes such as sanitation, supplier approval, and traceability.
3. Build Your Food Safety Plan & Prerequisite Programmes
Develop or update your HACCP plan (hazard analysis, critical control points, monitoring procedures, and corrective actions). Establish prerequisite programmes (PRPs) covering sanitation, personnel hygiene, pest control, allergen management, and supplier approval. Align these with regulatory requirements like FSMA and GMPs.
4. Document Control & Training Programme
Create controlled policies and procedures, assign a document number, and implement change‑control processes. Train employees at all levels on their roles, from sanitation to traceability. The SQF Code emphasises management commitment and employee awareness; training records are often audited.
5. Environmental Monitoring & Testing Plan
For manufacturing sites, design an environmental monitoring programme to verify that pathogens (e.g., Listeria or Salmonella) are under control. Include routine swabbing of equipment, drains, and zones, and plan for product testing where appropriate. Evidence of verification and corrective actions must be maintained.
6. Internal Audits & Mock (pre‑assessment) Audit
Conduct regular internal audits to verify compliance with your procedures and the SQF Code. Use trained auditors independent of the area being audited. Before the certification audit, consider a pre‑assessment audit by an external consultant to simulate the real process and identify any residual gaps.
7. Certification Audit & Corrective Action Closure
Select an accredited Certification Body (CB) and schedule your initial audit. The audit includes document review, interviews and a facility tour. If your operation involves any internal or outsourced laboratory testing for critical food safety parameters, the auditor may also review the technical competence of these processes; for this, adherence to standards like ISO 17025 can serve as strong evidence of reliable, validated testing methods.
Non‑conformances are categorised as minor (1‑point deduction), major (5‑point deduction), or critical (50‑point deduction). You start with 100 points; deductions lead to ratings: E (Excellent) 96–100, G (Good) 86–95, C (Complies) 70–85 and F (Failure) <70. A score of “C” triggers a six‑month surveillance audit; scores “E” or “G” result in annual recertifications. Corrective actions must be documented and verified within the timeframe set by the auditor.
8. Maintain & Continuous Improvement
After certification, maintain the system through routine audits, management reviews, and continuous improvement projects. The SQF Code requires unannounced audits at least once every three cycles, so always be audit‑ready. Monitor emerging risks (e.g., allergens, food fraud, cyber‑security) and update your risk assessments accordingly. Review consumer complaints, supplier performance and internal data to drive preventive action. Consider achieving the Quality Code to enhance product consistency and leverage the SQF Quality Shield.
What the Audit is like: Methods, Scoring, and Outcomes
An SQF audit typically spans one to three days, depending on facility size and complexity. Here’s what to expect:
Opening Meeting & Document Review
The auditor explains the agenda, clarifies the scope, and begins examining your documented food‑safety plan, procedures, supplier approvals, training records, and internal audit reports. Remote information and communications technology (ICT) may be used for part of this review, but at least 50 % of the audit must be conducted onsite, ensuring the auditor sees practices in action.
Facility Tour & Interviews
The auditor walks through the facility, observing hygienic practices, employee behaviours, equipment condition, and sanitary design. They will interview staff to confirm understanding and implementation of SOPs covering everything from handwashing to policies on facial hair and RPE are understood and followed.. Expect questions on allergen control, traceability, product identification, and environmental monitoring.
Non‑conformance Grading
Each clause of the SQF Code is assessed. Meeting the requirement earns zero deductions; a minor non‑conformance is a 1‑point deduction, a major is 5 points, and a critical is 50 points. Critical non‑conformances (e.g., evidence of gross contamination) lead to immediate audit failure. The overall score (out of 100) dictates your rating: Excellent (96–100), Good (86–95), Complies (70–85), or Fail (<70). The rating determines audit frequency: scores E and G require annual re‑certification; C results in a six‑month surveillance audit.
Post‑audit Process
You’ll receive a report detailing non‑conformances and due dates for corrective actions. Minor issues may require a written plan; majors require objective evidence of correction. Critical findings halt certification until the issue is resolved.
Pre‑audit Checklist
Before the auditor arrives, ensure the following items are complete:
- Scope defined and correct SQF modules selected.
- Documented Food Safety Plan (HACCP) and validated critical control points.
- Written prerequisite programmes (sanitation, allergen management, pest control, supplier approval).
- Up‑to‑date training records for all employees and evidence of competency.
- Calibration records for thermometers, scales, and other monitoring devices.
- Environmental monitoring schedule and trend analyses.
- Supplier approval documentation and certificates of analysis (COAs).
- Corrective action reports from internal audits and mock recalls.
Common Non‑conformances & How to Fix Them
Auditors often observe the same pitfalls across facilities. We have identified the following top issues and their remedies:
Poor document control: Outdated procedures on the shop floor or uncontrolled copies.
Fix: Centralise documents, assign revision numbers, and ensure only current versions are accessible. Train staff to remove obsolete documents.
Missing internal audits: Companies either skip internal audits or audit only parts of the facility.
Fix: Develop an annual internal audit schedule covering all code elements, conduct audits objectively, and implement corrective actions.
Temporary repairs not documented: For example, duct‑taped pipes or makeshift fixes that become permanent.
Fix: Document temporary repairs, identify their root cause, and track them to closure; build a maintenance plan to replace damaged equipment.
Equipment design and construction issues: Rusted or corroded surfaces, flaking paint, and improper welds create harbourage for pathogens.
Fix: Use food‑grade materials, schedule preventive maintenance, and implement hygienic design improvements.
Incomplete hazard analysis & flow diagrams: Some sites omit allergens or fail to map the entire process.
Fix: Conduct multidisciplinary hazard analyses that consider biological, chemical, physical, and allergenic hazards; update flow diagrams whenever the process changes.
Poor record maintenance: Missing or incomplete monitoring records.
Fix: Implement electronic or paper systems that prompt operators to record data in real time; verify records during internal audits.
Improper chemical storage: Hazardous chemicals stored near ingredients or packaging.
Fix: Segregate chemicals in designated areas with secondary containment; train staff on chemical management.
Facility condition & sanitation: Peeling paint, unsealed wall penetrations, pests, and inadequate cleaning.
Fix: Maintain the building envelope, seal gaps, implement pest‑control programmes, and validate sanitation effectiveness.
Conclusion
SQF certification is more than a compliance exercise; it’s a strategic investment in protecting consumers, meeting buyer expectations, and safeguarding your brand. By adopting a structured approach, you can achieve an excellent or good rating and keep costly non‑conformances at bay. The stakes are high: foodborne illnesses cause vast human suffering and cost companies millions of dollars. The good news is that SQF provides a clear roadmap and globally recognised assurance. Use the information and tools in this guide to start or strengthen your SQF journey. In doing so, you’ll not only satisfy retailer requirements but also build a culture where food safety and quality are integral to your company’s DNA.
FAQS
Is SQF Mandatory or Just Buyer-driven?
SQF certification isn’t legally mandatory, but it’s often required by major retailers and foodservice buyers. Achieving it demonstrates trust, reduces audit duplication, and helps you access high-value markets.
What is the Difference between SQF and HACCP?
HACCP is a preventive system identifying and controlling food safety hazards, while SQF builds on HACCP principles to create a comprehensive, third-party audited certification program recognized globally under GFSI.
How Often Will I Be Audited After Certification?
SQF sites are audited annually, with at least one unannounced audit every three certification cycles to verify continued compliance and ongoing system effectiveness.
Is Fundamentals Real Certification and GFSI-benchmarked?
Yes, the SQF Fundamentals Program issues genuine SQF certificates; however, it’s not GFSI-benchmarked. It serves as a structured starting point for small and medium food businesses progressing toward full SQF certification.







